Peptides for appetite control
DNF-10® is a hydrolysate fraction from Saccharomyces cerevisiae which acts on the gut-brain mediators of satiety, to lower calorie intake and reduce body fat mass. Benefits demonstrated after the first weeks. Supported with 4 published clinical trials. 500 mg/day.
DNF-10® - Key features
-
Patented ingredient
-
Demonstrated mechanism of action: gut-brain axis moderation of the appetite
-
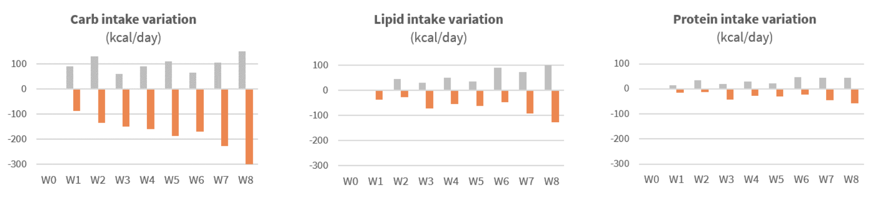
Significant reduction of calorie intake after first weeks of supplementation
-
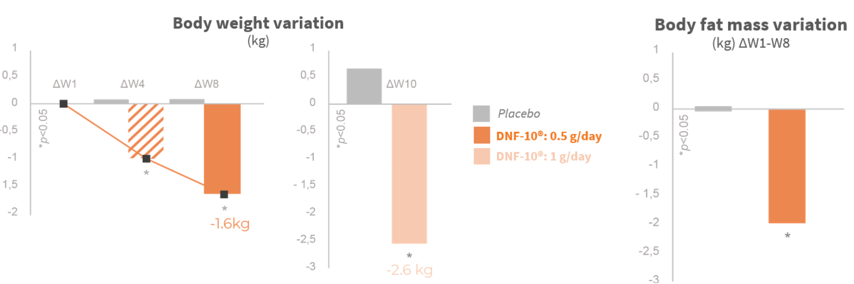
Significant weight loss after the first month
-
The weight lost is from fat mass
-
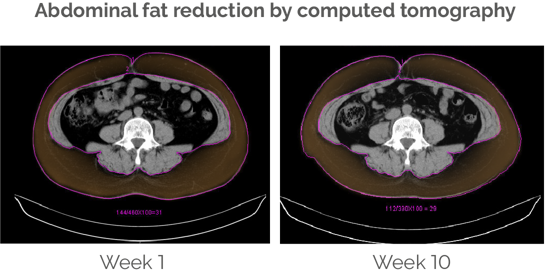
Abdominal fat and waist circumference decrease significantly
Science supporting DNF-10®
More than 10 years of research have been conducted to support the mechanism of action and clinical benefits of DNF-10® on body composition. The mechanism of action of DNF-10® on the mediator of satiety has been studied in vivo in animal models. The reduction of food intake and improvement of body composition have been confirmed with interventional human clinical trials.
More than 10 articles supporting the mechanism of action and health benefits of DNF-10® have been published.
Regulatory & Applications
GRAS self-affirmed with FDA notification GRN1033
Non-GMO project certified, Halal, gluten-free, suitable for vegetarians
Stability validated after heat treatment at 160°C/320°F for 30 min
References
CLINICAL TRIALS
Hong K. et al.; Progr. Nutr., 2015, 17:3:262-264
Jung E. et al.; Phytother. Res., 2009, 3(5):619-23
Jung E. et al.; Nutrition; 2014, 30:25-32
Jung E. et al.; Prev. Nutr. Food Sci.; 2017, 22(1):45-49
MECHANISTIC STUDIES
Park Y. et al.; Food Chemistry, 2013
Jung E.Y. et al., Ann. Nutr. Metab. 2012; 61(2):89-94
Jung E.Y. et al.; Journal of Health Science, 2011; 57(6), 532-539
This website is intended to provide information about Fytexia’s ingredients, used in various food/dietary supplement products around the world. It is only intended for business to business and to provide information to food/dietary supplement professionals and is not designed for the general public. Statements used on this website have not been evaluated by the Food and Drug Administration or any other competent authority. Products are not intended to diagnose, treat, cure or prevent any disease.