Digestive comfort
Benegut® is a patented botanical extract from shiso leaves (Perilla frutescens) combining antispasmodic effect and action on tight junction leakage to reduce the root causes of functional dyspepsia. Benegut® is clinically proven to contribute to a significant reduction of intestinal discomfort. Supported with an acute and a chronic clinical trial. 300 mg/day.
Benegut® - Key features
-
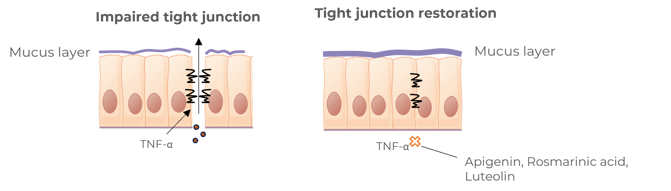
Contributes to the reinforcement of tight junctions of cells
-
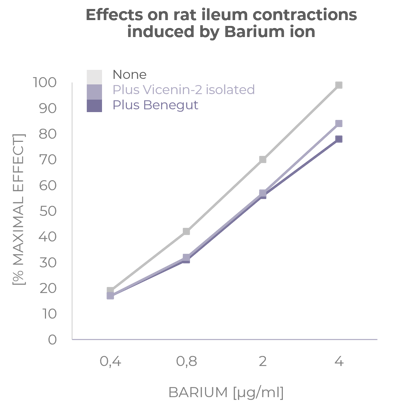
Provides an antispasmodic effect
-
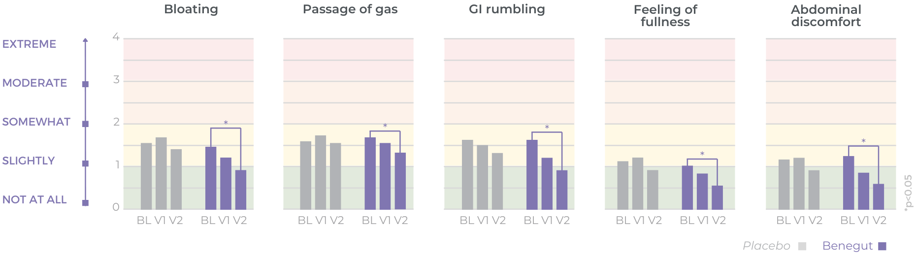
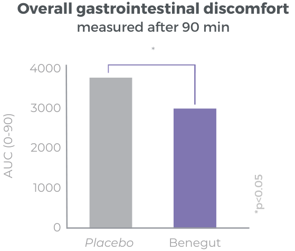
Clinically proven to rapidly reduce overall gastrointestinal discomfort
-
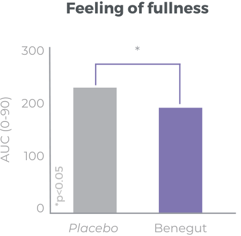
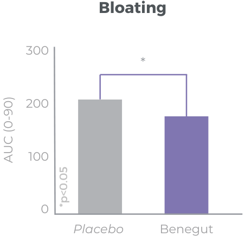
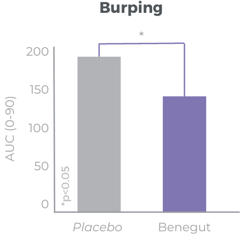
Improves bloating, feeling of fullness and abdominal comfort
Science supporting Benegut®
Benegut® is targeting digestive comfort and functional dyspepsia symptoms. Its mechanism of action on exploring antispasmodic effects and preventive effects on tight junction leakage, some of the causes of functional dyspepsia, have been studied in-vivo and in-vitro. The clinical benefits of Benegut® have been demonstrated in a chronic and an acute gold-standard clinical studies.
Regulatory & Certifications
Non-GMO, Halal, Kosher, gluten-free, suitable for vegetarians
References
CLINICAL TRIALS
2023 Publication pending
Buchwald-Werner S. et al. BMC Complement Altern Med. 2014; 14:173
MECHANISTIC STUDIES
Verspohl J. et al. Phytomedicine. 2013; 427–431
Buchwald-Werner S. et al. Agro Food Industry Hi Tech. 2016; vol. 27(5)
PATENT
WO2013079623. Vicenin 2 and derivatives thereof for use as antispasmodic and/or prokinetic agent.
TRADEMARKS
The trademark Nutrigut® must be used for the following territories: Argentina, Australia, European Union, New Zealand, Switzerland, Turkey, Ukraine.
This website is intended to provide information about Fytexia’s ingredients, used in various food/dietary supplement products around the world. It is only intended for business to business and to provide information to food/dietary supplement professionals and is not designed for the general public. Statements used on this website have not been evaluated by the Food and Drug Administration or any other competent authority. Products are not intended to diagnose, treat, cure or prevent any disease.