Bone health
MBP® is a whey protein fraction supported by mechanistic and clinical studies demonstrating its efficacy in reinforcing bone mineral density for women of all ages. 40 mg/day.
MBP® - Key features
-
Inhibits bone resorption through cysteine protease inhibition
-
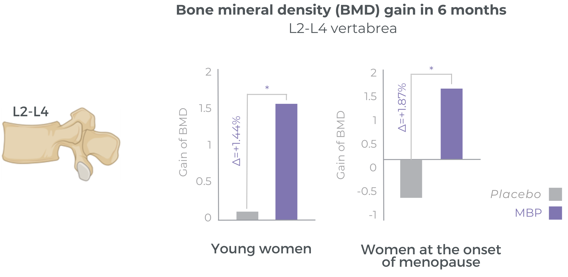
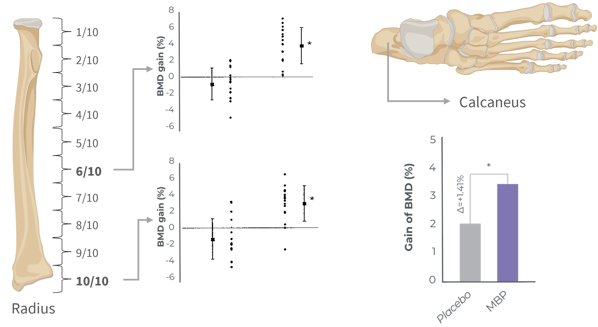
Clinically proven to increase bone mineral density by inhibiting bone resorption
-
Bone homeostasis is significantly improved after 3 months
-
Bone mineral density increases significantly after 6 months
Science supporting MBP®
Several clinical studies are supporting the benefits of MBP® on bone mineral density support. Double-blind, randomized, and placebo-controlled, these studies are designed to study different populations of women of all ages from younger age to post-menopause.
More than 10 articles supporting the mechanism of action and health benefits of MBP® have been published.
Regulatory & Certifications
Non-GMO, Halal, Kosher, gluten-free, suitable for vegetarians.
GRAS status - Notice No. GRN 000196
Japanese FOSHU registration 1276: "This product with MBP, which is effective to increase bone density, is suitable for people conscious of bone health"
South Korea Health Ingredient registration 2015-16: "May help to maintain healthy bone"
Australia / New Zealand - Claims available for food products: maintenance of normal bones/healthy bones // maintenance of bone mineralisation // maintenance of bone mineral density
References
MECHANISTIC STUDIES
Takada et al.; BBRC; 1995
Takada et al.; Int. Dairy Journal; 1997
Yamamura et al; BBRC; 1999
Yamamura et al.; BBRC; 2000
Takada et al.; Nutr. Aspec. Osteoporosis; 2001
Toba et al;Bone; 2001
Matsuoka et al.; Biosci. Biotechnol. Biochem.; 2002
CLINICAL TRIALS
Aoe et al; Osteoporosis Int; 2005
Uenishi et al; Osteoporosis Int; 2007
Aoe et al; Biosci. Biotechnol. Biochem; 2001
Yamamura et al; Biosci. Biotechnol. Biochem; 2002
This website is intended to provide information about Fytexia’s ingredients, used in various food/dietary supplement products around the world. It is only intended for business to business and to provide information to food/dietary supplement professionals and is not designed for the general public. Statements used on this website have not been evaluated by the Food and Drug Administration or any other competent authority. Products are not intended to diagnose, treat, cure or prevent any disease.