Immune & respiratory health
Promunel® is a brown propolis extract obtained from the patented Multi Dynamic Extraction process, guaranteeing a characterised and standardised full spectrum of polyphenols. Promunel® contributes to support immune and respiratory health. Promunel® is clinically proven to improve the comfort of the upper respiratory tract within 3 days.
Promunel® – key features
-
Brown propolis extract, obtained with the patented M.E.D.™ process which preserves the complete polyphenolic complex as occuring naturally in raw propolis
-
Demonstrated immunomodulatory role through macrophage polarisation - Published patent application
-
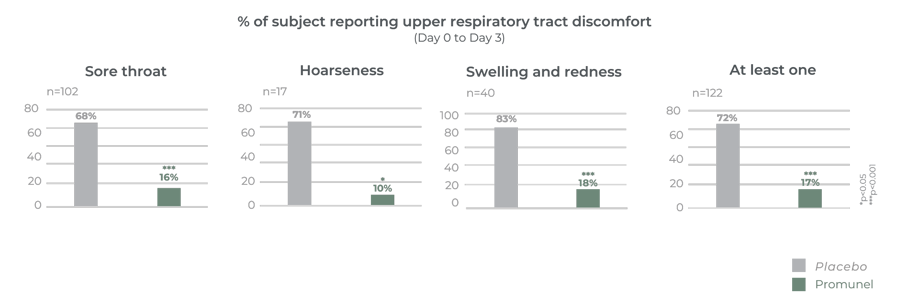
Clinically proven on 295 subjects to reduce the incidence of upper respiratory discomforts and shorten the average number of days with discomforts.
-
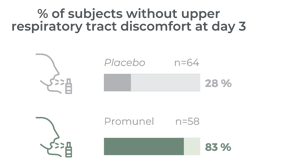
Clinically proven on 122 subjects to reduce the discomfort of upper respiratory tract within 3 days of supplementation
Science supporting Promunel®
It has taken many years to develop Promunel®, transforming raw propolis into an advanced ingredient. Traditionally propolis has a complex composition and natural variability but this research has enabled us to produce a characterised and standardised propolis extract. The mechanism of action and clinical benefits of Promunel® have been demonstrated in a study of 122 subjects suffering from upper respiratory tract discomfort.
Regulatory & Certifications
Non-GMO, Halal, Kosher, gluten-free, suitable for vegetarians
Available as organic
References
PROCESS
Zaccaria et. al. Materials, 2019
MECHANISTIC STUDIES
Zaccaria et. al. Nutrients, 2017
Garzarella et al. Biomedecine & Pharmacotherapy, 2022
CLINICAL TRIALS
Esposito et al. Phytomedicine, 2020
This website is intended to provide information about Fytexia’s ingredients, used in various food/dietary supplement products around the world. It is only intended for business to business and to provide information to food/dietary supplement professionals and is not designed for the general public. Statements used on this website have not been evaluated by the Food and Drug Administration or any other competent authority. Products are not intended to diagnose, treat, cure or prevent any disease.



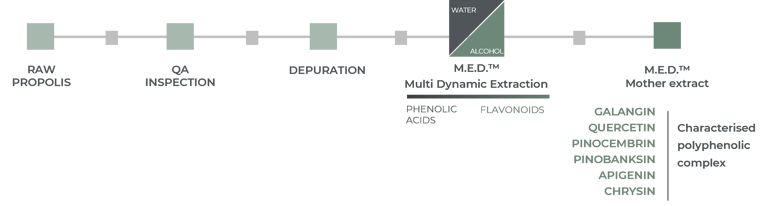 The M.E.D.TM process used to produce Promunel® allows the extraction of the complete, intact and identified polyphenolic complex. It ensures that at least 25% of the polyphenols in Promunel® are represented by the six main flavonoids of propolis: Galangin, Quercetin, Pinocembrin, Pinobanksin, Apigenin, Chrysin.
The M.E.D.TM process used to produce Promunel® allows the extraction of the complete, intact and identified polyphenolic complex. It ensures that at least 25% of the polyphenols in Promunel® are represented by the six main flavonoids of propolis: Galangin, Quercetin, Pinocembrin, Pinobanksin, Apigenin, Chrysin.